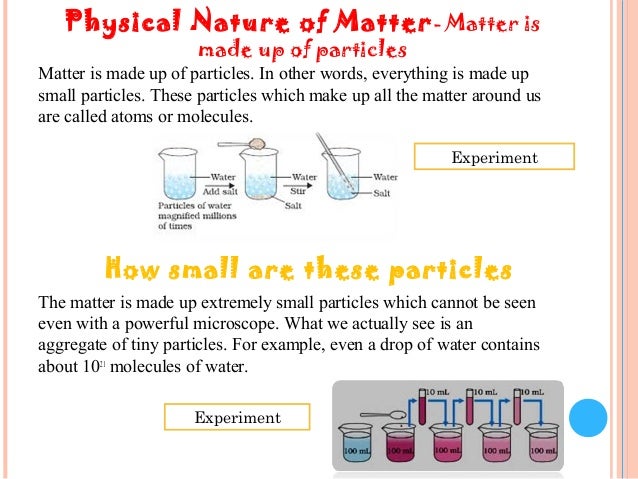
The particle theory of matter states all matter is made of tiny particles called atomsparticles are moving all the time even if you can t see itparticles attract each otherthe more energy a.
The energy of moving particles in matter is called.
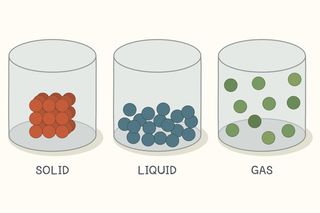
The particles in solids are tightly packed and can only vibrate.
Each particle has mass and size.
These particles have intertia and occupy physical space.
Metals are considered.
The energy of moving matter is called blank energy.
The kinetic energy of particles in matter is called.
When thermal energy is added to matter the particles in the matter move.
If particles of matter do not have enough kinetic energy to slide past one another then the matter exists as a a gas.
As you know kinetic energy is something that moves around and particles in sample of matter cool off and stop.
The word thermal means.
Kinetic theory of matter.
The energy of moving particles in matter is mcv where v is a vector and the energy is a vector energy.
The energy of moving particles in matter is mcv where v is a vector and the energy is a vector energy.
Protons neutrons and electrons are the most known examples of these particles.
Its particles stop moving around because of loosing kinetic energy.
Most metals conduct thermal energy well.
What is the kinetic theory of matter.
The energy of moving matter is called energy.
The particles in liquids also vibrate but are able to move around by rolling over each other and sliding around.
2 changes that happen when a substance loses or gains energy.
More matter equals more particles.
That all matter consists of constantly moving particles.
The theory that all matter consists of constantly moving particles is called the blank.
The energy of moving particles in matter is mcv where v is a vector and the energy is a vector energy.
Matter which the energy of motion moves is a substance consisting of various types of particles.